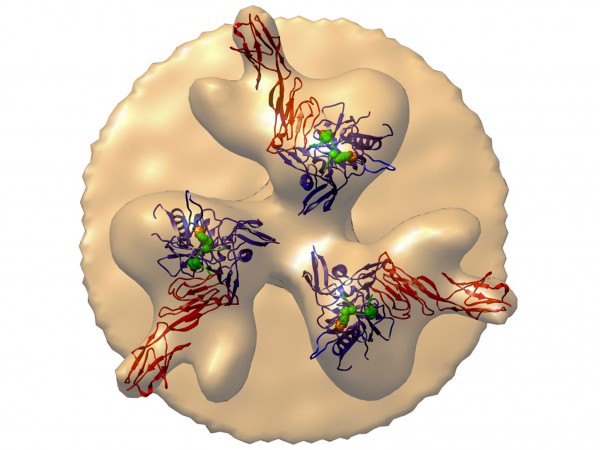
An aerial view of HIV’s surface proteins (blobs) shows how eCD4-Ig can bind to the virus and “neutralize” it. (adapted from Michael Farzan et al., Nature, 2015)
For 30 years, researchers have struggled to determine which immune responses best foil HIV, information that has guided the design of AIDS vaccines and other prevention approaches. Now, a research team has shown that a lab-made molecule that mimics an antibody from our immune system may have more protective power than anything the body produces, keeping four monkeys free of HIV infection despite injection of large doses of the virus.
Intensive hunts are under way for natural HIV antibodies that can stop—or “neutralize”—the many variants of the constantly mutating AIDS virus. Researchers have recently found several dozen broadly neutralizing antibodies (bNAbs) that are highly potent and work at low doses. But viral immunologist Michael Farzan of the Scripps Research Institute in Jupiter, Florida, and 33 co-workers have recently taken a different strategy, building a novel molecule based on our knowledge of how HIV infects cells. HIV infects white blood cells by sequentially attaching to two receptors on their surfaces. First, HIV’s own surface protein, gp120, docks on the cell’s CD4 receptor. This attachment twists gp120 such that it exposes a region on the virus that can attach to the second cellular receptor, CCR5. The new construct combines a piece of CD4 with a smidgen of CCR5 and attaches both receptors to a piece of an antibody. In essence, the AIDS virus locks onto the construct, dubbed eCD4-Ig, as though it were attaching to a cell and thus is neutralized.
continue reading / weiterlesen / ادامه مطلب