Men who carry the human papillomavirus (HPV) have a higher risk of HIV infection. New data published in the Journal Aids indicate that infection with HPV is associated with a higher risk of infection with HIV, a precursor to Aids.
While the links between HPV infection and HIV in women have been widely studied, data about similar associations among men is limited.
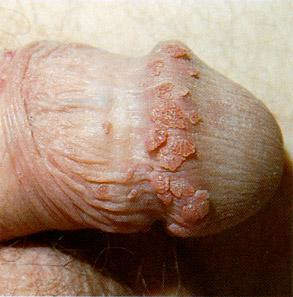
photo: the Genital Warts Solution
The study, conducted in Kisumu, Kenya by researchers from the University of North Carolina, adds to mounting evidence showing a higher risk of HIV infections among individuals with genital HPV infections.
Men in the study with at least one occurrence of HPV infection faced double the risk of acquiring HIV, while those with three or more HPV infections had more than three times the risk of developing HIV. This pattern was consistent in both circumcised and uncircumcised men.
“With the current observational studies, it remains unclear whether there is a role for HPV vaccination to reduce the risk of HIV acquisition,” said Anne Rositch, the lead researcher and an assistant professor at the University of Maryland School of Medicine.
However, HPV vaccination has been shown to prevent HPV-associated cancers. A series of random clinical trials were conducted in Kisumu between 2002 and 2007.
Samples were taken from the penile shaft, foreskin, glans and coronal sulcus. The researchers controlled for the effect of circumcision, the main intervention in the study. The study revealed that only samples from the glans were associated with increased risk of HIV
The aim of the study was to determine the effectiveness of circumcision in reducing cases of HIV infection.
A total of 61 men developed HIV during the trial. HIV is one of the five leading killer diseases in Kenya. Sub-Saharan Africa leads the world in HIV infections and deaths as the virus claims between 1.5 million and 1.9 million lives each year according to the World Health Organisation.
HPV infection is the primary cause of several genital cancers, including cervical cancer in females and anal cancer in both men and women. Along with Aids-related malignancies, epidemic levels of HPV infection contribute to the growing cancer burden experienced by nations in less-developed countries.
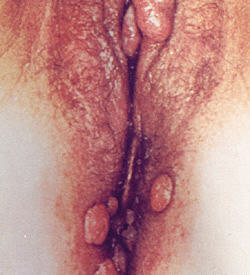
photo: the Genital Warts Solution
The National Institutes of Health has recommended that women receive a single dose of HPV vaccine to protect themselves against the virus.
Vaccination status
“It will be important to link future vaccination status with HIV incidence databases on the population level to examine the impact of HPV vaccination coverage on the incidence of new HIV infections,” said Jeniffer Smith, an associate professor at the Gillings School of Global Public Health.
The findings raise the possibility that vaccination against the HPV virus could help curb the HIV pandemic.
By CHRISTABEL LIGAMI, Special Correspondent (posted 2013 December 14)
Source: The East African
GlaxoSmithKline (GSK) announced today that the European Commission has granted marketing authorisation for its cervical cancer [Human papillomavirus bivalent (types 16 and 18) vaccine, recombinant] as a two-dose schedule for girls aged 9 to 14. This is the first time a cervical cancer vaccine has been approved as a reduced dosing schedule and signifies potential for greater vaccination coverage rates and improved cervical cancer protection worldwide.
photo: PULSE
Worldwide, cervical cancer claims an average of one life every two minutes with an estimated 275,000 deaths from the disease each year.1 Virtually all cases of cervical cancer are caused by a virus called human papillomavirus (HPV).2 There are more than 100 known types of HPV, 3 of which at least fifteen can cause cervical cancer.4 HPV 16 and 18 are the most common cancer-causing virus types and account for approximately 70% of all cervical cancer cases globally.5 Up to 80% of women worldwide will acquire an HPV infection in their lifetime and almost 40% of these infections will be with a cancer-causing virus type.2,6,7
The vaccine is already approved in the EU for use in females from the age of 9 years, administered according to a three-dose schedule (vaccination at months 0,1 and 6) for the prevention of premalignant genital (cervical, vulvar and vaginal) lesions and cervical cancer causally related to certain oncogenic Human Papillomavirus (HPV) types. The two-dose schedule (0, 6 months) will apply to vaccination of girls aged 9-14. The three-dose schedule remains in the label for girls and women aged 15 years and above.
Outside of the EU, Cervarix two-dose schedule in girls aged 9-14 years is already approved in twelve countries (including Panama, Guatemala, Honduras, El Salvador, Haiti, Suriname, Chile, Guyana, Nigeria, Ghana, Pakistan and Bangladesh).
Cervarix is also approved in the US. For the full US Prescribing Information and EU Patient Information Leaflet, which includes information on the approved use of Cervarix™, please visit GlaxoSmithKline (GSK).
